CORONAVIRUS
KSL Special Report: COVID-19 Vaccine
Nov 27, 2020, 8:09 PM | Updated: Dec 4, 2020, 12:26 pm
SALT LAKE CITY, Utah – Leading health experts agree, three promising vaccine candidates could help us get life back to normal, as Utah and the country face growing numbers of COVID cases.
Moderna, Pfizer and AstraZeneca all announced over the last couple weeks that their vaccines in clinical trials are safe and effective. The companies released this data in press releases, which is required by the FTC to avoid insider trading.
That data now faces a lot of scrutiny from the FDA and CDC, but many hope at least one will be available for front line healthcare workers as early as this month.
In this COVID-19 Vaccine special report, KSL TV breaks down what is known about each vaccine and what the process for rolling out the vaccine will look like.
What is known about each vaccine?
The latest vaccine to make headlines is the AstraZeneca–Oxford university vaccine. Data shows 70% effectiveness in large scale tests. We learned after the press release came out that the second group received a smaller dose by accident. That anomaly conflated the numbers, with the smaller group seeing 90% effectiveness and the larger group seeing about 62%. Now experts with the WHO want more research amid these questions on the data. That compares with last month’s results from Pfizer and Moderna – both more than 90% effective.
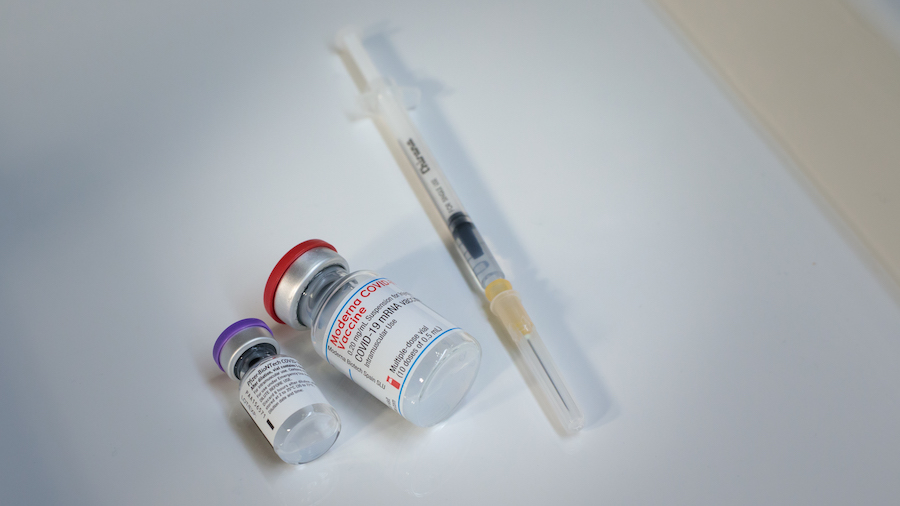
Because of the fragility of the vaccine–Pfizer’s vaccine needs to be frozen at minus 70 degrees celcius. Moderna needs to be frozen at minus 20 degrees celcius, which is something a more normal freezer could handle. Both can be kept refrigerated for days, and at room temperature for hours. The AstraZeneca–Oxford vaccine can be stored at normal refrigerator temperature, which may make it easier to get to people around the world.
In all three trials, some people who received the vaccine experienced an immune response. They had fatigue, fever, body chills, but none of them lasted more than a day and none of them required medical attention, just some rest and Tylenol. One person in the AstraZeneca trial developed a serious, but treatable, disease but researchers do not believe that had anything to do with the vaccine.
How does the vaccine work?
The Moderna and Pfizer vaccines are a revolutionary type of vaccine, which uses mRNA to train your body to fight the virus. Instead of injecting parts of a dead virus into your system, the mRNA vaccine teaches your body to create a spike protein that is found on the COVID virus. When your body is fighting the COVID virus, it creates antibodies which attach to the spike protein, blocking the virus from entering your cells. The vaccine teaches your own body how to make that spike protein, detached from the virus so you can’t get sick, and now your body knows how to make antibodies. Researchers started developing this technology during the SARS-COV-1 outbreak in the early 2000s.
All three vaccines closest to being approved for the public require two doses–either three or four weeks apart. Dr. Tamara Sheffield from Intermountain Healthcare is involved with the CDC’s Advisory Committee on Immunization Practices and says that second dose is very important.
“With this first COVID vaccine, your body produces a pretty good amount of antibodies, similar to a moderate amount of what would happen if you’ve got the virus yourself,” she said. “But with a second dose, you actually produce a lot more of the antibodies to kind of a high level of what people who are ill produce and that is a much more protective piece.“
In order to reach herd immunity, public health researchers think we need would need about 70% of people with some level of immunity. And they say that’s just not an option without a vaccine.
BYU Public Health associate professor Chantel Sloan says, “The vaccine is our only acceptable path to herd immunity, We’ve had a dramatic increase in infections in Utah and around the country. But we’re still only at about, I think around 5% of Utahns have been actively infected. And at 5%, we’re seeing our ICUSs, our hospitals get overrun, our medical staff stretched to the brink.”
How do vaccine trials work?
According to the CDC, before even giving the vaccine to humans, researchers go through extensive pre-clinical and animal trials to determine what will be safe and effective.
Once they reach a clinical development stage–where they actually start testing vaccines in humans–there are several phases.
In Phase One, vaccine is given to a small group of healthy volunteers. This test phase is meant to show that the vaccine is safe, and to begin to understand how human bodies react to it. In this phase, researchers also look at what doses will be safest and most effective based on how it elicits an immune response in the participants blood.
Phase two continues to monitor safety, but includes many more people–including the vaccine’s target group–like elderly folks and front line workers in the case of COVID. Researchers continue watching for effectiveness and side effects.
In phase three, scientists give the vaccine widely, to thousands of volunteers. Researchers usually add a control group getting a placebo here. In this phase, researchers are specifically looking at how effective the vaccine is in keeping people from getting sick in the real world.
Phase Four is an on-going, long-term phase that happens after the vaccine is approved for public use and scientists continue to follow volunteers to watch for long term safety and efficacy.
How have trials been able to move so much faster than normal?
The short answer: money.
One researcher told the BBC much of the time spent in other vaccine development is waiting on money, trial participants and infrastructure.
He said, “The process is long, not because it needs to be and not because it’s safe, but because of the real world.”
Right now, every medical researcher is working on COVID vaccines and treatments, where their research is normally spread across hundreds of different areas.
Finally, Dr. Sheffield says the sheer number of people getting sick and being exposed to the virus gets researchers to their targets faster.
“The more people you have ill with the condition you’re trying to prevent, the faster you can get the data that you need to tell whether the vaccine is working or not.“
Who will get the vaccine and when?
Utah is hoping to start vaccinating people as early as the end of next month.
Sometime in December healthcare workers at the state’s 5 largest hospitals who are working with COVID patients regularly will be able to get the vaccine. Late December or January: other healthcare workers like workers at hospitals, clinics, long-term care facilities, home health care, as well as first responders and public health workers will get the vaccine. February and March: Residents in long-term care facilities and remaining Essential workers. March through July will see a role out of the rest of people, focusing first on people over 65, racial and ethnic minority groups at higher risk, people with underlying health conditions and workers like teachers, childcare professionals, and personal care workers. As we work through all of those higher risk people, the vaccine will start to be available for everyone else.
Right now, the vaccines have been tested in healthy adults, a small group of teenagers, people over the age of 65, and people with underlying health concerns, like heart and lung disease. Vaccines haven’t been tested on children under the age of 12–but those trials are expected to begin soon. They also have not–and may not ever–be tested in pregnant women and people with immunocompromising conditions — like those going through chemo. People who had COVID already should also get the vaccine as scientists still don’t know how long our immunity will last.
What should we do in the mean time?
Public health officials say it’s very important we keep up with the things we know work while we’re waiting for a vaccine. We know already from other countries that have gotten COVID under control without a vaccine that masking, distancing, hygiene, testing and contact tracing works. State health officials also encourage people to get a flu shot to ease the strain on our healthcare system.